- Biotechnology group>
- Basic Research>
- Mechanism for regulating gene expression in Corynebacterium glutamicum
Basic Research
Mechanism for regulating gene expression in Corynebacterium glutamicum
Mechanism for regulating gene expression in Corynebacterium glutamicum
1. Transcriptional regulation mechanism
1.1. Transcription factor for sugar uptake gene
1.2. Transcription factor for sugar metabolism gene
1.3. Global regulator
1.4. Sigma factor
1.5. Profiling of gene expression under oxygen deprivation
2. Post-transcriptional regulation mechanism
1. Transcriptional regulation mechanism
A transcription factor binds to the promoter region of its target gene, thereby activating (a transcriptional activator) or repressing (a transcriptional repressor) the synthesis of mRNA by RNA polymerase (transcription).
Various types of transcription factors recognize specific DNA sequences and their activity is controlled in their specific manners. The overlaps and the interactions between the regulatory modules (signal for regulation - transcription factor - target gene) form a complicated network. It is thought that transcription factors act as sensors for various intra-/extra-environmental signals which are integrated through the microbial gene regulatory network into the cellular response and adaptation to fluctuating environmental conditions.
It is estimated that there are at least 150 transcription factors encoded by the genome of Corynebacterium glutamicum, and a great advance has recently been made in characterization of these factors. Our research group has identified and characterized a number of transcription factors for regulating cellular metabolism primarily from the point of view of the regulation of sugar metabolism and the response to oxygen deprivation.
Future Microbiol. 5: 1475-1481. 2010. (Review)
1.1. Transcription factor for sugar uptake gene
<Analysis of sugar utilization system>To achieve a cost-effective bioprocess, it is necessary to develop a microorganism which is able to metabolize various biomass-derived sugars simultaneously. To this end, identification of the sugar utilization systems and understanding of their regulation are critical. Our research group has investigated the sugar utilization systems in Corynebacterium glutamicum R. We found that this strain possesses glucose-, fructose-, sucrose-, and two β-glucoside- phosphoenolpyruvate (PEP), carbohydrate phosphotransferase systems (PTS) and identified the transcription factors involved in the regulation of these genes. In addition, we identified a C. glutamicum strain that can utilize arabinose and characterized its utilization genes and their transcription factor. We have also investigated the systems for utilization of other carbon sources such as organic acids and alcohols in this bacterial species.
Mol. Microbiol. 92: 356-368. 2014.
Appl. Microbiol. Biotechnol. 97: 8139-8149. 2013.
Appl. Microbiol. Biotechnol. 89: 1905-1916. 2011.
Appl. Microbiol. Biotechnol. 91: 1375-1387. 2011.
Appl. Environ. Microbiol. 75: 3419-3429. 2009.
Appl. Environ. Microbiol. 75: 3461-3468. 2009.
Appl. Environ. Microbiol. 74: 5290-5296. 2008.
Appl. Microbiol. Biotechnol. 76: 1347-1356. 2007.
Microbiology. 149: 1569-1580. 2003.
Biochem. Biophys. Res. Commun. 289: 1307-1313. 2001.
Our research group has been trying to expand the sugar utilization capability of Corynebacterium glutamicum R for efficient utilization of biomass. In addition to glucose, mannose, fructose, and sucrose, which are metabolized by the wild-type strain, a point mutation on the β-glucoside pts gene enables the strain to use cellobiose. Moreover, we have succeeded in constructing the genetically engineered strain capable of utilizing xylose and arabinose in addition to PTS sugars simultaneously.
Appl. Microbiol. Biotechnol. 85: 105-115. 2009.
Appl. Microbiol. Biotechnol. 81: 691-699. 2008.
Appl. Microbiol. Biotechnol. 77: 1053-1062. 2008.
Appl. Environ. Microbiol. 72: 3418-3428. 2006.
Microbiology. 149: 1569-1580. 2003.
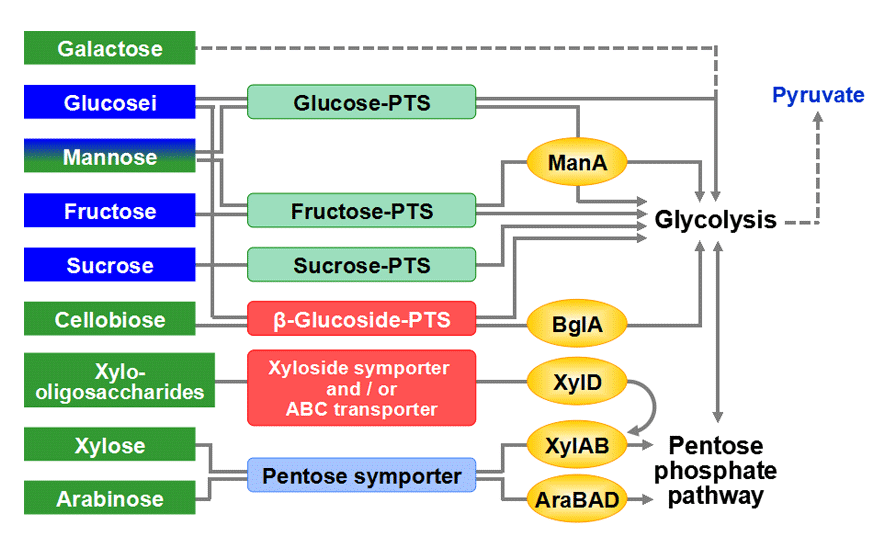
<Sugar utilization systems in Corynebacterium glutamicum R>
Sugar transport by phosphoenolpyruvate : carbohydrate phosphotransferase system (PTS)
Among sugars from biomass, glucose, mannose, fructose, sucrose, and cellobiose are transported into the cell by the PTS in Corynebacterium glutamicum R. A unique feature of the PTS is that it catalyzes uptake of the sugars by coupling carbohydrate translocation and phosphorylation. The phosphoryl group from PEP is sequentially transferred to enzyme I, HPr, EIIs and finally to the substrate as it is translocated across the membrane. In addition to carbohydrate uptake, the PTS regulates expression of many catabolic genes depending on its phosphorylation state.
<Transcription factors for the PTS>For the improvement of the biomass sugar utilization capability of Corynebacterium glutamicum, understanding the regulatory mechanism of the pts genes is essential. We identified several transcription factors for the pts genes. Expression of the pts genes is induced in the presence of the PTS sugar. It was demonstrated that the sugar-phosphate generated by metabolism of the PTS sugar inactivates the transcription factor SugR, and results in the increased expression of the pts genes. Complex regulation of the pts genes by various transcription factors can make this bacterium cope with diverse environments.
Appl. Microbiol. Biotechnol. 89: 1905-1916. 2011.
J. Bacteriol. 193: 349-357. 2011.
Microbiology. 155: 3652-3660. 2009.
Microbiology. 154: 264-274. 2008.
Appl. Microbiol. Biotechnol. 78: 309-318. 2008.
Microbiology. 149: 1569-1580. 2003.
Biochem. Biophys. Res. Commun. 289: 1307-1313. 2001.
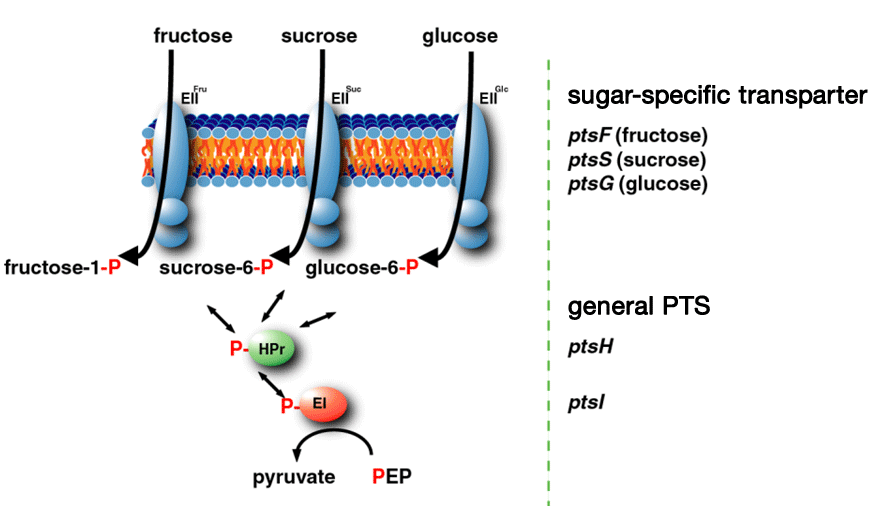
<Phosphoenolpyruvate : carbohydrate phosphotransferase system (PTS)>

<Transcriptional regulation of the pts genes in Corynebacterium glutamicum>
1.2. Transcription factor for sugar metabolism gene
Knowledge on regulatory mechanisms of expression of genes involved is fundamental for enhancement of sugar metabolism by metabolic engineering strategies. We have sought to characterize transcription factors involved in regulation of central metabolic genes encoding especially enzymes of the glycolytic and pentose phosphate pathways in Corynebacterium glutamicum. We focus on transcriptional regulatory mechanisms of these enzymes because the glycolytic pathway followed by fermentative lactate dehydrogenase plays a key role in maintenance of cellular redox balance (NADH/NAD+) under oxygen deprivation, and the pentose phosphate pathway plays a key role in NADPH generation and utilization of C5 sugars derived from cellulosic biomass.
J. Bacteriol. 191: 968-977. 2009.
Appl. Microbiol. Biotechnol. 83: 315-327. 2009.
J. Bacteriol. 191:4251-4258. 2009.
Appl. Microbiol. Biotechnol. 81: 291-301. 2008.
Microbiology. 154: 3073-3083. 2008.
J. Mol. Microbiol. Biotechnol. 15: 264-276. 2008.
Microbiology. 153: 2190-2202. 2007.

<Transcriptional regulation of genes involved in glycolysis, fermentative lactate dehydrogenase, and pentose phosphate pathway in Corynebacterium glutamicum>
1.3. Global regulator
A global regulator is defined as a transcription factor controlling several genes involved in different physiological reactions and metabolic pathways. Due to diversity of its target genes, modification of gene and/or activity of a global regulator generally has great influence on phenotypes.
Some of transcription factors regulating sugar metabolic genes in Corynebacterium glutamicum have been shown to act as global regulators controlling versatile cellular functions including anaerobic metabolism and cell division. We have sought to understand physiological roles of the transcription factors by identifying a set of their direct target genes (regulon) using genome-wide analyses including transcriptome analysis and ChIP-chip analysis. A transcription factor and its regulon comprise a regulatory module. Interaction among the regulatory modules constitutes a transcriptional regulatory network, which plays a key role in systematic regulation of biological reactions and metabolic pathways. These genome-wide transcriptional regulatory mechanisms are applied to design strains for efficient utilization of various carbon sources and optimization of bio-production processes.
J. Bacteriol. 193: 4123-4133. 2011.
Microbiology. 157: 21-28. 2011.
J. Bacteriol. 191: 968-977. 2009.
J. Bacteriol. 190: 8204-8214. 2008.
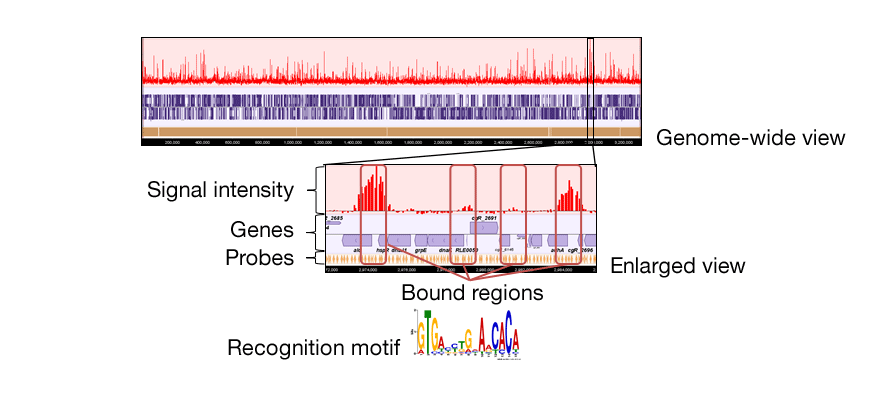
<ChIP-chip analysis>
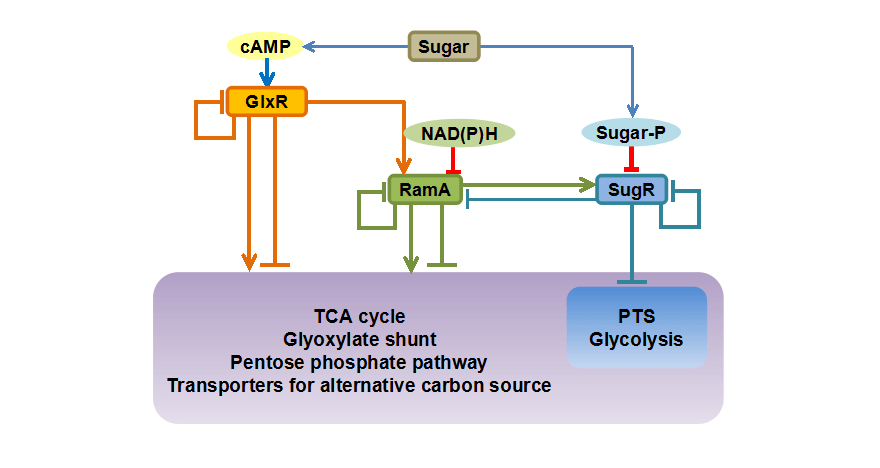
<Transcriptional regulatory mechanism among transcription factors>
1.4. Sigma factor
A sigma (σ) factor forms RNA polymerase holoenzyme with core enzyme consisting of αββ'ω subunits. A σ factor is responsible for recognition and binding of holoenzyme to promoters and core enzyme catalyzes mRNA synthesis using DNA as a template. A bacterium possesses more than one σ factor, each of which binds to specific promoter and initiates transcription. A bacterium modifies gene transcription by switching σ factors to adapt to environmental changes.
Seven σ factors are encoded in the genome of Corynebacterium glutamicum. The principal σ factor SigA is involved in housekeeping genes transcription and essential for growth. The group 2 σ factor SigB exhibits high amino acid identity to SigA, but is not essential. The remaining σ factors SigC, SigD, SigE, SigH, and SigM belong to extracellular function (ECF) σ family, which includes σ factors important for environmental stress responses.
We have sought to understand physiological roles of each σ factor by determining consensus promoter sequence and identifying its regulon. Knowledge obtained will help understand molecular mechanisms of stress response and be applied to development of useful promoters.
J. Bacteriol. 191: 2964-2972. 2009.
Appl. Environ. Microbiol. 74: 5146-5152. 2008.
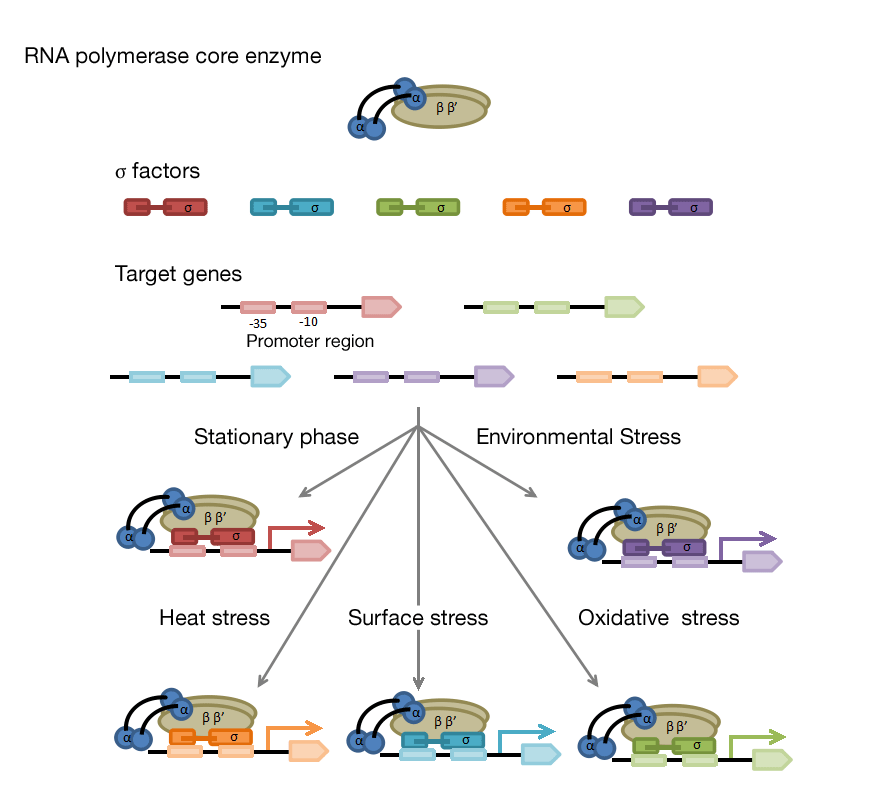
<Switching of genes transcribed by switching σ factor>
1.5. Profiling of gene expression under oxygen deprivation
Our research group has developed bioprocesses based on the high productivity of organic acids by packed Corynebacterium glutamicum cells under oxygen deprivation. Transcriptome analysis based on 3080 genes on the genome reveals that 161 genes are upregulated and 221 gene are downregulated under oxygen deprivation conditions as compared with aerobic conditions. To effectively use the gene expression profile for the basis of metabolic engineering, we have characterized transcription factors for the genes up/down-regulated, implying the complicated cross talk between the regulatory systems in response to various environmental signals under the conditions.
FEBS J. 279: 4385-4397. 2012.
Biosci. Biotechnol. Biochem. 76: 1952-1958. 2012.
Microbiology. 158: 975-982. 2012.
Appl. Microbiol. Biotechnol. 90: 1051-1061. 2011.
Appl. Environ. Microbiol. 76: 5488-5495. 2010.
Microbiology. 156: 1335-1341. 2010.
J. Biol. Chem. 284: 16736-16742. 2009.
Appl. Microbiol. Biotechnol. 83: 315-327. 2009.
J. Bacteriol. 191: 4251-4258. 2009.
Appl. Environ. Microbiol. 74: 5146-5152. 2008.
Appl. Microbiol. Biotechnol. 81: 505-513. 2008.
Microbiology. 153:2491-2504. 2007.
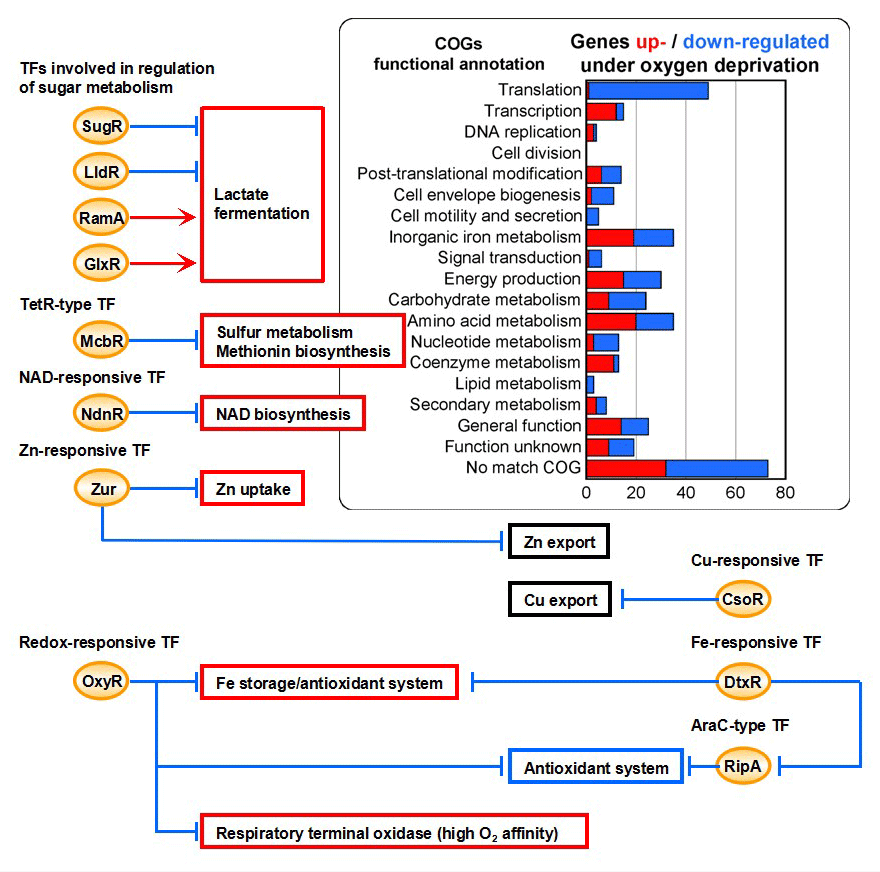
<Diverse physiological roles and transcription factors (TFs) for genes up-/down-regulated under oxygen deprivation>
<Anaerobic nitrate respiration>Corynebacterium glutamicum had been thought to be an obligate aerobe for a long time. However, we recently reported that it can grow under anaerobic conditions using nitrate as an electron acceptor, and that the nar operon involved in the nitrate respiration is controlled by a novel-type transcription factor ArnR and a global regulator GlxR. In addition, our transcriptome analysis revealed that the SOS genes involved in an inducible DNA repair and damage tolerance system are upregulated under anaerobic nitrate respiration conditions. Furthermore, we observed that genes encdoding various enzymes for maintenance of the intracellular NADH/NAD+ ratio are up-/down-regulated under the conditions, and the characterization of transcription factors for these genes is in progress in our research group.
J. Bacteriol. 193: 1327-1333. 2011.
Microbiology. 157: 21-28. 2011.
J. Bacteriol. 190: 3264-3273. 2008.
Mol. Microbiol. 67: 597-608. 2008.
Appl. Microbiol. Biotechnol. 75: 889-897. 2007.
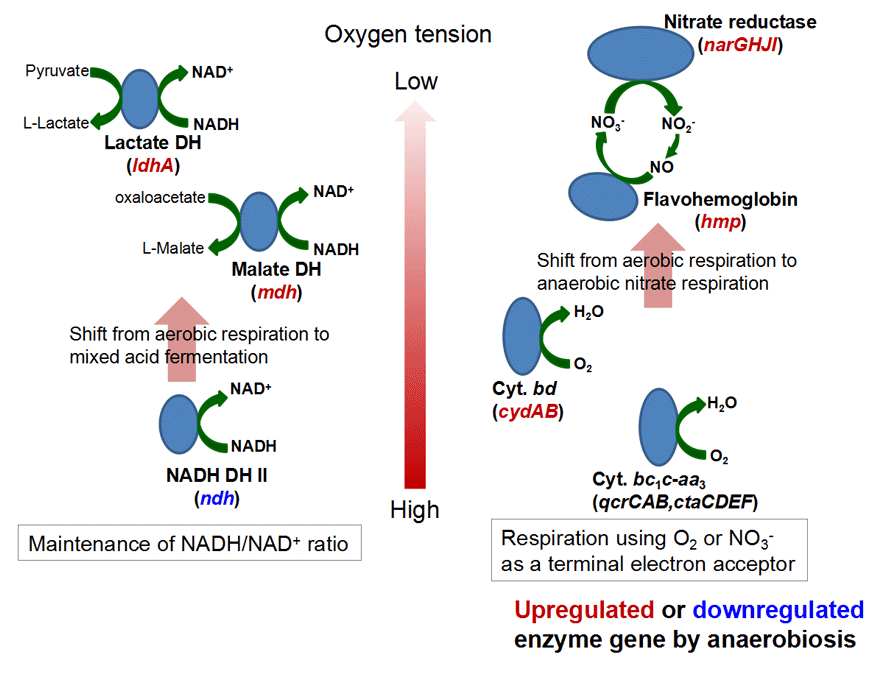
<Shift from aerobic respiration to anaerobic nitrate respiration/mixed acid fermentation in Corynebacterium glutamicum>
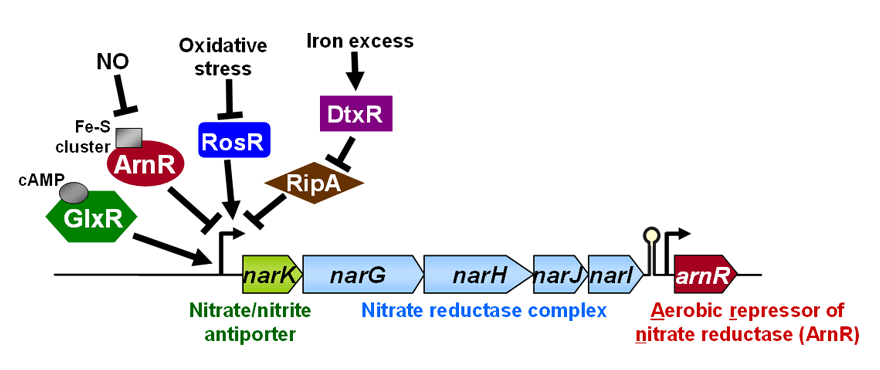
<Transcriptional regulatory systems for genes involved in anaerobic nitrate respiration in Corynebacterium glutamicum>
2. Post-transcriptional regulation
To control metabolic activity, it is necessary to optimize the quantity of enzymes. To date, many studies focusing on transcriptional regulation of metabolic enzymes are performed extensively and many tools are produced for optimization of expression of metabolic enzymes. However the regulation of gene expression in vivo is performed not only at transcriptional level but also at post-transcriptional level. It is expected that combination of transcriptional and post-transcriptional regulation systems leads to a new tool that has higher quality. To accomplish development of such a powerful tool, we are studying the mechanism of post-transcriptional regulation of gene expression.
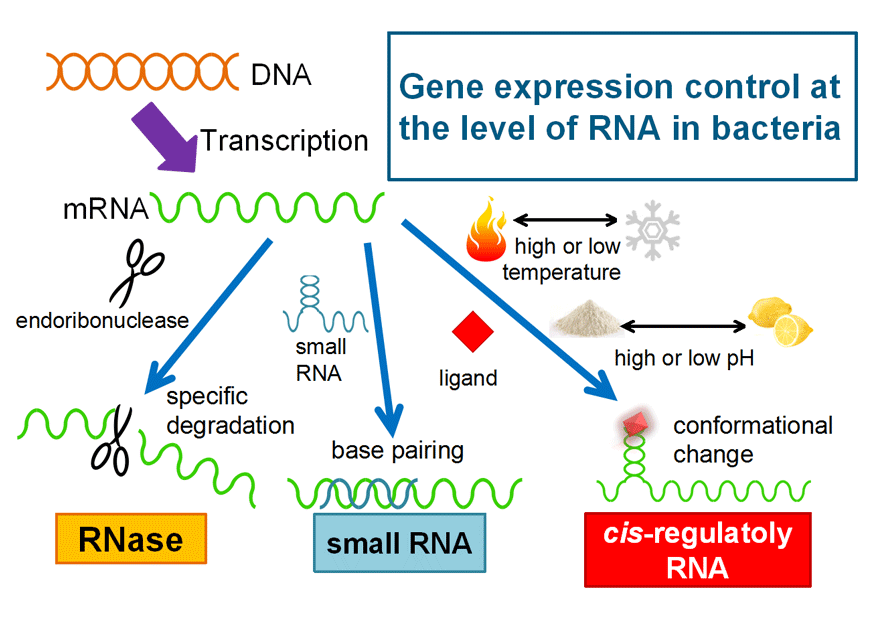
<Regulation of gene expression at mRNA level in bacteria>
<Regulation of expression of the β-glucoside pts genes>Regulation of genes encoding the β-glucoside PTS differs from those of other pts genes in that it is controlled at a transcription elongation step. Transcription of the bgl gene, encoding β-glucoside PTS, terminates at a terminator present at 5′ untranslated region (UTR) of the mRNA. In the presence of β-glucoside, binding of a transcription antiterminator protein BglG to 5′-UTR of bgl mRNA prevents the formation of terminator structure, results in the elongation of transcripts to the end of the gene. The RNA binding activity of BglG is controlled by the PTS-mediated phosphorylation/dephosphorylation, therefore, the expression of the bgl gene is increased by the presence of extracellular β-glucoside.
Microbiology. 155: 3652-60. 2009.
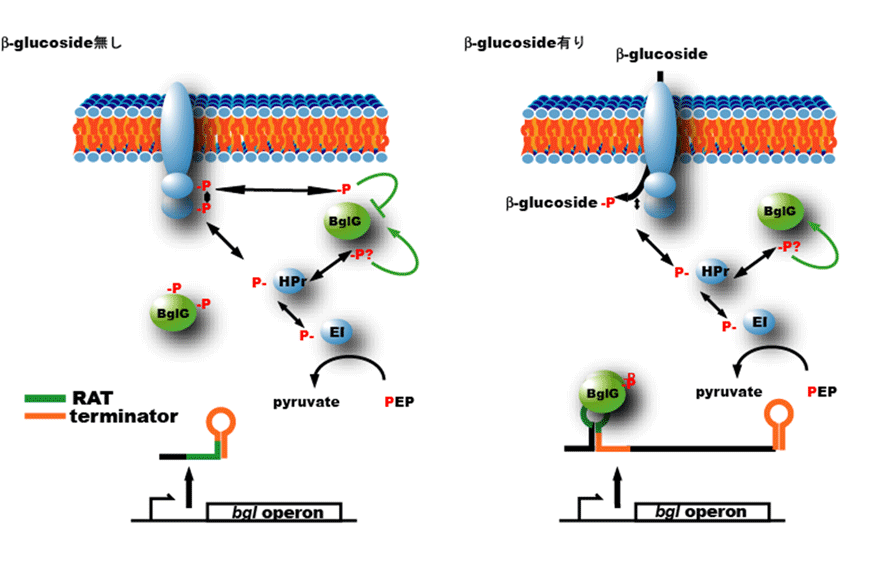
<Transcription antitermination regulation of the bgl operon encoding β-glucoside PTS>
