 |
 |
|
|
Basic Research Purpose The purpose of basic research is to ascertain through laboratory testing the physical properties of bedrock in order to translate various log results obtained at the Iwanohara site; for elucidating the residual gas saturation rate by clarifying the trap mechanism; and predicting the long-term behavior of CO2 by identifying the chemical reaction between CO2 and surrounding bedrock. Seismic Wave and Specific Resistance Tests Using Sandstone Samples Using samples of sandstone, which is the typical bedrock used as the reservoir in aquifer storage, the change of seismic waveforms (P-wave) before and after CO2 injection was measured, and a decline of its velocity was confirmed. The obtained results were utilized in the analysis of seismic tomography. Schematic of test setup
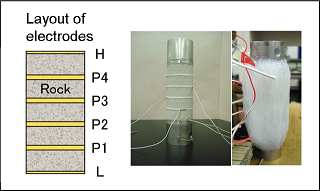
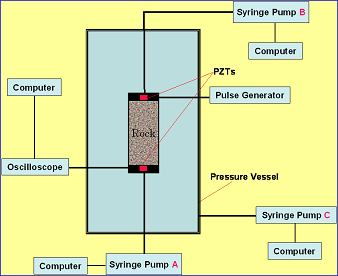 Seismic waveforms before and after CO2 injection 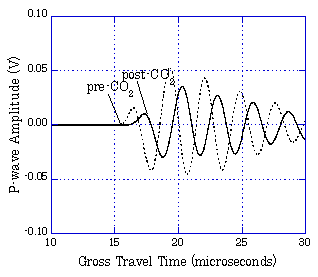 Using the same sandstone samples, the specific resistance (electric resistance per unit volume) between electrodes was measured before and during CO2 injection. Because CO2 does not conduct electricity, the specific resistance will increase if CO2 flows in. In this test, it was observed that the specific resistance gradually increased when CO2 was injected from the lower side of the sandstone sample. Layout of electrodes in specific resistance measurement
 Time-lapse change of specific resistance by CO2 injection 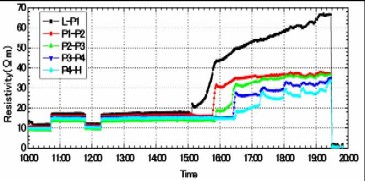 From these tests, it was confirmed that CO2 injected into the aquifer can be monitored through the changes of seismic wave velocity and specific resistance. Evaluation of CO2 Saturation by Sonic Logging Based on Gassmann Theory Based on the Gassmann theory (a theory that explains the change of seismic wave velocity when the voids of a porous material are filled with water or gas) which has been employed in the field of oil and natural gas, the CO2 saturation was evaluated using the sonic logging results obtained at the Iwanohara test site. The relationship between P-wave velocity and CO2 saturation was investigated using the data obtained at depths of 1,115m, 1,116m, and 1,117m at the Iwanohara site. From the results, it was revealed that if the CO2 saturation is 20% or lower, CO2 saturation can be estimated using the sonic logging results and the Gassmann theory. The obtained results are in agreement with the results of NMR logging (nuclear magnetic resonance logging: a method to measure the water amount and the void size in the formation by giving a magnetic pulse to the hydrogen nucleus in the formation and measuring its response) which was conducted separately. This made possible a quantitative evaluation of the saturation of CO2 which is injected into the reservoir. Relationship between P-wave velocity and CO2 saturation
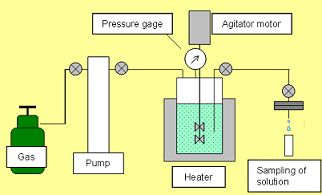
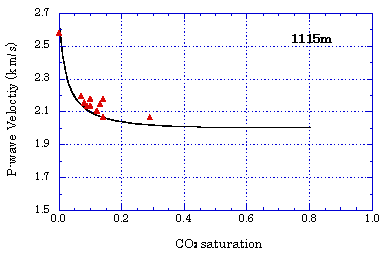 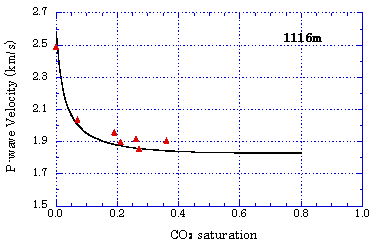 Evaluation of Mineral Fixation by Geochemical Reaction The calcium concentration in the solution was measured by conducting a reaction test using core samples and the formation water taken at the Iwanohara test site. It was found that, with the lapse of time, calcium dissolved out from the core samples and the calcium concentration in the solution increased. With the decrease of hydrogen ions as calcium dissolves out, the formation water is neutralized and it tends to dissolve CO2. If the formation water returns to near neutrality, calcium carbonate will be deposited (such as calcite). Hence, there is the possibility to eventually produce mineral fixation which is a stable storage form of CO2. Schematic of laboratory reaction test setup
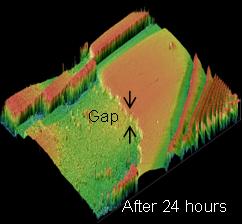
 Change of calcium concentration 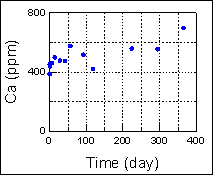 Using a phase contrast electron microscope, the dissolution rate of an anorthite was measured. This revealed that the dissolution of minerals due to CO2 is temperature dependent. It was found that the dissolution rate of minerals 1,000m below the Iwanohara site, where the temperature is around 50 °C, is approximately five times faster than that near the ground surface where the temperature is approx. 25 °C. Dissolution of mineral surface captured by a phase contrast electron microscope
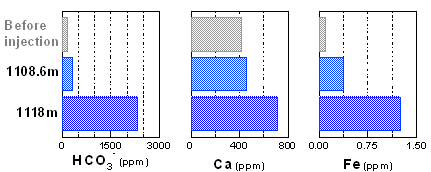
 Dissolution rate of anorthite 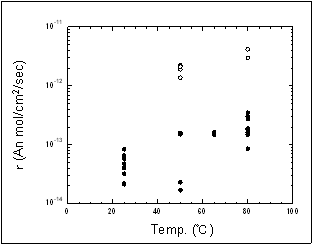 At the Iwanohara site, formation water was sampled at a depth of 1,118m where CO2 is stored and at a depth of 1,108.6m where no change was found via both specific resistance logging and sonic logging. From such analysis, it was found that bicarbonate ions (HCO3) increased at 1,118m, indicating that CO2 was dissolving at that depth. Calcium and iron were also found to increase, which means that a reaction between CO2 and minerals was taking place. Based on the aforementioned, it is considered that mineral fixation will advance quicker than predicted at the Iwanohara site. Change in the composition of formation water at the Iwanohara test site
 Achievements
Future Challenges
|
|
|||||
|
|
