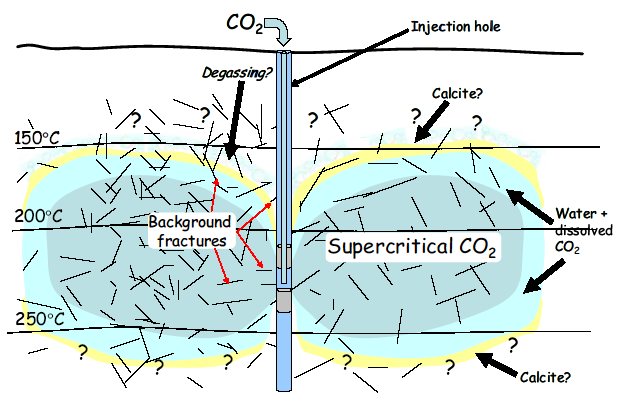
Concept of CO2 fixation system
1. Purpose
Reaction rate of CO2 and some mineral increases and
solubility of carbonate minerals decreases under high temperature condition. In
the case of CO2 storage into geothermal field, CO2 fixation as carbonate minerals is
expected. Once carbonate minerals precipitate forming a sealing layer, CO2 storage will be possible
under the
sealing layer. Then this study is carried out to estimate the possibility of
CO2 storage technology into geothermal filed.

Concept of CO2 fixation system
2. Results
Assessment of CO2 storage capacity and cost in geothermal field
We discussed the possibility and application of the CO2 fixation system in Japan. To assess the practical use, we selected the geothermal areas excluding the national park area and main CO2 generate facilities (thermal power plants and cement factories) 20km or more away. Several geothermal areas for practical use exist in Hokuriku and Kyusyu area of Japan. Total capacity of CO2 storage was estimated at 1.18 billion tons CO2 on the basis of assumed rock porosities (average: 4%) and the solubility of CO2 (3 wt%). The cost was evaluated at 5136 yen/ton- CO2 in the case of storage rate of 1 million tons CO2 per year and transport distance of 20km.Laboratory experiment of CO2-water-rock interaction under hydrothermal conditions
Field experiment in Ogachi geothermal field
Field experiments were performed to investigate CO2-water-rock interaction in Ogachi geothermal field. CO2 dissolve water (4.3 wt%) was injected into the bored hole and granodiorite (Ogachi rock) was soaked. The temperature was 208 °C, and soaking time were 16.5 hours. After the soaking, the dissolution of anhydrite was observed as well as the laboratory experiment.Simulations of CO2-water-rock interaction under hydrothermal conditions
Copyright(C) Research Institute of Innovative Technology for the Earth (RITE). All rights reserved.